By Wendy Rasmussen
Natural products industry is under increasing pressure to increase analytical testing of raw materials and finished products. This not only includes contaminants (such as heavy metals, pesticides, and mycotoxins), but also identification and nutrition labeling.
The DSHEA (Dietary Supplement Health & Education Act) created the Office of Dietary Supplements in 2002. The Dietary Supplement Quality Assurance Program, is a collaboration between the NIH’s ODS and the NIST. The program consists of Exercises in which several samples are sent out to participating labs throughout the year, and the results are then analyzed in a report. This is a completely voluntary exercise, and it gives a laboratory a great opportunity to check their results against a certified value and also against those of their peers. Even though participation is not part of a regulatory certification, the choice to participate greatly increases confidence and credibility.
And since the NIST are working closely with the NIH’s Office of Dietary Supplements, I asked the ODS if they could describe the relationship:
“The DSQAP is part of the ODS Analytical Methods and Reference Materials Program. We’ve been funding them to produce supplement matrix reference materials since about 2002. A few years ago, we discovered that NIST had QA programs for other commodities, and we thought that a dietary supplement program would complement the reference materials already being produced and added funding to the inter agency agreement we already had in place with them. While we are a funder for the NIST program, it could be described as more of a collaboration than a strict funding relationship. NIST pretty much matches our funding with internal funding. “
The NIST website http://www.nist.gov/mml/analytical/dsqaprogram.cfm also does a good job of describing the program in more detail. If you would like to participate in the DSLQAP, they would love to have more participants, just send them an email to the address on the above webpage.
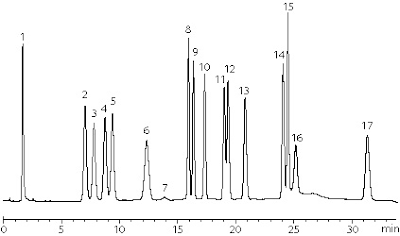
This past September, the NIST hosted a workshop in Bethesda, MD which brought together participants from several sample exercises. This was a great opportunity for labs and companies to come together to discuss and learn. Pickering Laboratories participated in the most recent Exercises by analyzing Aflatoxin in peanut samples.
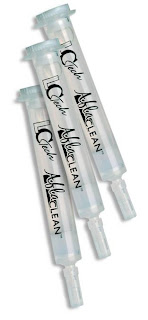
We used our Photochemical reactor (UVE) and our AflaCLEAN Immunoaffinity columns for the sample cleanup. The results can be found on our new application note and in a presentation given to the group at the workshop.
We gave a quick presentation to the group on Thursday morning, detailing our method and results. We would love to share our talk titled simply “Aflatoxin Analysis”. Just send us an email to support@pickeringlabs.com and we’ll send you a copy!
The Photochemical derivatization of Aflatoxins has been shown to be a very rugged and sensitive method for analyzing low-levels of the toxins. And the Immunoaffinity columns can be used to cleanup a very wide variety of sample matrices. Indeed, there have been several publications showing the results using Photochemical Derivatization.
We are now distributing Mycotoxin Immunoaffinity products for Ochratoxin and Aflatoxin. The performance and batch-to-batch reproducibility of the columns is exceptional and far exceeds that of other manufacturers. The columns can be used for any matrix, from wine and juice, to nuts and grains, to herbs and spices. Contact Sales for more information.
Definition of Acronyms:
NIH – National Institutes of Health
ODS – Office of Dietary Supplements, part of NIH
NIST – National Institute of Standards and Technology
DSLQAP – Dietary Supplement Laboratory Quality Assurance Program, formed by the ODS in collaboration with NIST
AHPA – American Herbal Products Association
AOAC – Association of Analytical Chemists
Laszlo Torma has also contributed a very nice piece about our Membership in the AHPA on our blog.