Pickering at the LAPRW 2017
This year, David Mazawa and Rebecca Smith traveled to and participated in The 6th Latin-American Pesticide Residue Workshop: Food and Environment (LAPRW 2017), which took place from May 14-17 in San Jose, Costa Rica. The location was perfect for discussing the global impact of environmental residues: Costa Rica protects over a quarter of all its land in national parks, wildlife refuges and reserves. And the country boasts nearly 6% of the entire world’s biodiversity!

Costa Rica is home to Macaws and hundreds of other beautiful birds.
This workshop continued building on the success of the previous five LAPRW workshops by keeping scientists and regulators informed about the advances in pesticide residue regulation in food and the environment, methodologies of analysis, instrumentation, risk analysis, monitoring, and quality control assurance of laboratories.

Three toed sloth, hanging out.
Pickering Laboratories, Inc. is proud to sponsor and support this great conference. We would like to thank the organizers: Centro de Investigación en Contaminación Ambiental (CICA) and different units of the Universidad de Costa Rica (UCR) and the Servicio Fitosanitario del Estado (SFE) of the Ministerio de Agricultura y Ganadería de Costa Rica (MAG). Their hard work and dedication really showed in the quality of the conference and this workshop wouldn’t have been possible without them.

David at the Pickering Booth, reviewing the exciting technical program!
After an exciting week of technical talks and networking with scientists from around the world, we came back to the lab with great contacts, and several new ideas for how to best serve our international customers.
We look forward the next LAPRW workshop at Iguazu Falls, Brazil!

 Pickering Laboratories exhibited at the 67th Pittcon annual conference, which was held March 5-9 in Chicago this year. Pittcon is the leading exhibition for laboratory science and new technology in food safety, life science and emerging markets. As we have for the last 30 years, Pickering Laboratories exhibited as the leader in Post-Column applications and technology.
Pickering Laboratories exhibited at the 67th Pittcon annual conference, which was held March 5-9 in Chicago this year. Pittcon is the leading exhibition for laboratory science and new technology in food safety, life science and emerging markets. As we have for the last 30 years, Pickering Laboratories exhibited as the leader in Post-Column applications and technology.


 Broader acceptance of medical cannabis use increases the need for analytical methods capable of determining the active compounds of cannabis as well as for methods to detect contaminations, such as pesticide residues, mycotoxins and traces of organic solvents. Cannabinoids are a class of terpenophenolic compounds that are associated with the pharmacological activity of cannabis. Cannabinoids exist in the plant mainly as carboxylic acids that are not physiologically active. They are converted to neutral analogs by light and heat while in storage or during the preparation of edible products. Acids are also converted to neutral analogs during GC analysis, which often causes differences in results when comparing with HPLC methods.
Broader acceptance of medical cannabis use increases the need for analytical methods capable of determining the active compounds of cannabis as well as for methods to detect contaminations, such as pesticide residues, mycotoxins and traces of organic solvents. Cannabinoids are a class of terpenophenolic compounds that are associated with the pharmacological activity of cannabis. Cannabinoids exist in the plant mainly as carboxylic acids that are not physiologically active. They are converted to neutral analogs by light and heat while in storage or during the preparation of edible products. Acids are also converted to neutral analogs during GC analysis, which often causes differences in results when comparing with HPLC methods.
 Pickering Labs would like to congratulate the winners of our last newsletter’s Shifting Retention Times–Carbamates Quiz: David Green from Pepperdine University, Jeff Fan from Cumberland Valley Analytical Services, Karissa Scroggins from North Coast Laboratories, Jim Balk from Nebraska DHHS Public Health Environmental Laboratory, Narjes Ghafoori from LA County Agricultural Commissioner Weights & Measure Environmental Toxicology Lab, Tom Schneider from Suffolk County Water Authority, and Ms. Widchuda Meeim from Thailand Bureau of Quality Control of Livestock Products.
Pickering Labs would like to congratulate the winners of our last newsletter’s Shifting Retention Times–Carbamates Quiz: David Green from Pepperdine University, Jeff Fan from Cumberland Valley Analytical Services, Karissa Scroggins from North Coast Laboratories, Jim Balk from Nebraska DHHS Public Health Environmental Laboratory, Narjes Ghafoori from LA County Agricultural Commissioner Weights & Measure Environmental Toxicology Lab, Tom Schneider from Suffolk County Water Authority, and Ms. Widchuda Meeim from Thailand Bureau of Quality Control of Livestock Products.
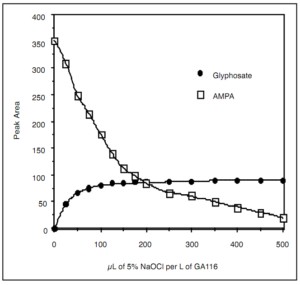
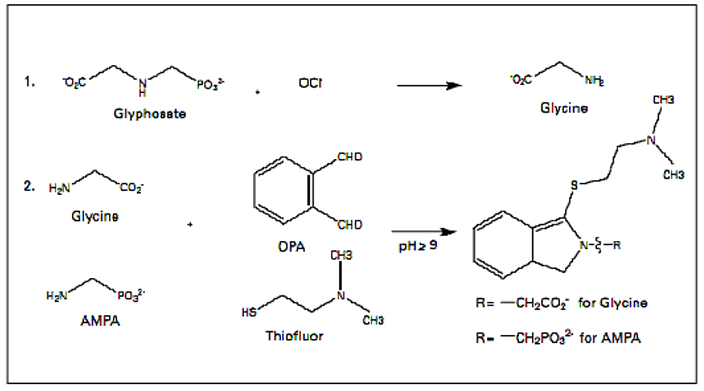

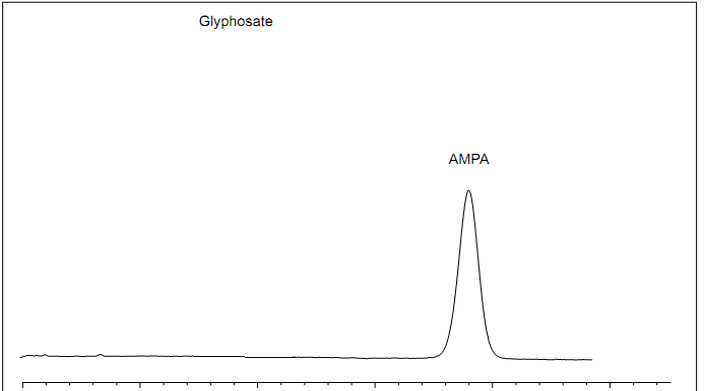
 Events surrounding Glyphosate testing in foods continue to evolve with the latest stories “France banning Glyphosate sales in consumer nurseries” and “California requiring labeling Glyphosate as a carcinogen.” We also want to discuss the EU- wide petition to ban Glyphosate.
Events surrounding Glyphosate testing in foods continue to evolve with the latest stories “France banning Glyphosate sales in consumer nurseries” and “California requiring labeling Glyphosate as a carcinogen.” We also want to discuss the EU- wide petition to ban Glyphosate.
 Pickering Laboratories has reported earlier that we developed and validated a post-column method for analysis of Theanine in tea (Camellia sinensis) dietary ingredients and supplements. Theanine is the main naturally occurring amino acid found in tea and it is responsible for a number of health benefits of tea as well as for its savory pleasant taste.
Pickering Laboratories has reported earlier that we developed and validated a post-column method for analysis of Theanine in tea (Camellia sinensis) dietary ingredients and supplements. Theanine is the main naturally occurring amino acid found in tea and it is responsible for a number of health benefits of tea as well as for its savory pleasant taste.
