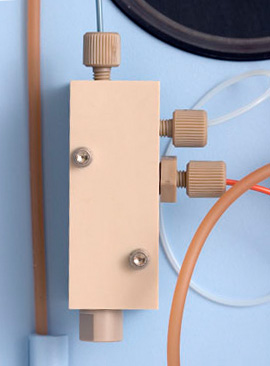
Cleaning and Reassembly of the Over-pressure Relief Valve
-
Remove the tubing connections to the Mixing Manifold. Use a 3/32” hex driver to remove the 2 screws holding the Mixing Manifold to the chassis. Use a 3/8” wrench to remove the end cap and discard the old Over-pressure Relief Valve Cartridge. Ultrasonicate the Mixing Manifold for at least 30 minutes. Rinse well with DI water.
-
Connect the outlet of your HPLC pump to the Mixing Manifold inlet and pump 100% water at 0.5mL/min to verify the Mixing Manifold is not clogged. If the Mixing Manifold is still clogged after cleaning in an ultrasonicating bath, replace the Mixing Manifold Assembly (PN 1452-0040).
-
Turn off the HPLC flow and make sure there is no pressure on the Mixing Manifold. Insert the new OPRV cartridge, green side down, and screw on the end cap to 20”lbs of torque. To approximate this level of torque, first finger tighten, then tighten an additional 1/8-1/4 turn with a 3/8” wrench.
- To verify the opening pressure of the Over-pressure Relief Valve, plug the two side inlets of the Mixing Manifold and turn on the HPLC pump to 0.5mL/min. Allow the pressure to slowly rise. The Over-pressure Relief Valve should open around 485psi. If the opening pressure is too low, tighten an additional 1/8 of a turn with a 3/8” wrench.
David Mazawa
david.mazawa@pickeringlabs.com
Technical Support Chemist
Pickering Laboratories, Inc.
1280 Space Park Way
Mountain View, CA 94043 USA
Phone: (650)694-6700 ext. 710
Fax: (650)968-0749








